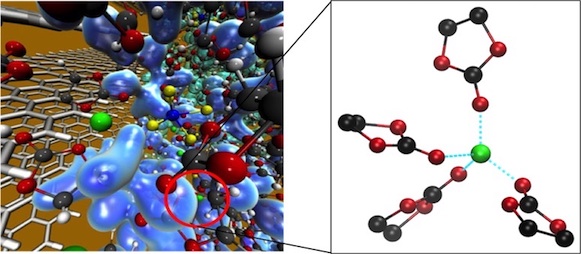
Lithium-ion battery performance is strongly influenced by the ionic conductivity of the electrolyte, which depends on the speed at which Li ions migrate across the cell and relates to their solvation structure. The choice of solvent can greatly impact both solvation and diffusivity of Li ions. We use first principles molecular dynamics to examine the solvation and diffusion of Li ions in the bulk organic solvents ethylene carbonate (EC), ethyl methyl carbonate (EMC), and a mixture of EC/EMC. We find that Li ions are solvated by either carbonyl or ether oxygen atoms of the solvents and sometimes by the PF6 anion. Li ions prefers a tetrahedrally-coordinated first solvation shell regardless of which species are involved, with the specific preferred solvation structure dependent on the organic solvent. In addition, we calculate Li diffusion coefficients in each electrolyte, finding slightly larger diffusivities in the linear carbonate EMC compared to the cyclic carbonate EC. The magnitude of the diffusion coefficient correlates with the strength of Li ion solvation. Corresponding analysis for the PF6 anion shows greater diffusivity associated with a weakly-bound, poorly defined first solvation shell. These results may be used to aid in the design of new electrolytes to improve Li-ion battery performance. This work was published in Journal of Physical Chemistry B.
